Protect patients from wholesalers who break the supply chain
American Medicine Is Only Safe Because of Our Closed Supply Chain
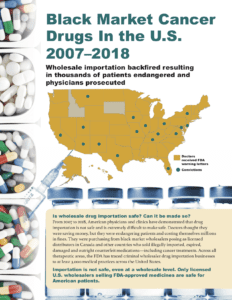
Since 2012, the U.S. government has identified and prosecuted wholesalers who have enriched themselves by importing drugs—some of them counterfeit, and all of them non-FDA-approved—from all over the world. These wholesalers misrepresented the source of their products, claiming that they were FDA-approved or that they came from "safe" countries such as Canada or countries in the European Union. Their drugs reached more than 3,000 medical practices in all therapeutics areas: oncologists, neurologists, dermatologists, orthopedists, obstetricians, general practitioners, and more.
We will never know the full effect of these drugs on Americans. We do know that patients received cancer medicine that had no active ingredient, and sought care in emergency rooms to deal with painful side effects.
In 2019, 10 states have pushed the U.S. government to approve drug importation from so-called “safe” countries, and the government is planning to accommodate them. However, even these “safe” countries have problems with counterfeit medication in their supply chains:
- In 2017, the Medicines and Health Regulatory Agency (MHRA) described the United Kingdom as a target of and a hub for counterfeit medicine sales.
- In 2018, Finland was forced to issue a warning after counterfeit versions of the cancer medication Velcade were discovered in Finnish hospitals.
Importation and Supply Chain Break News
Doctor Prosecutions
Learn more and share these resources
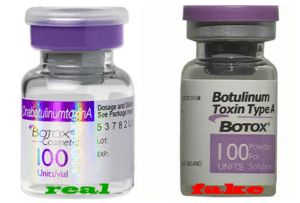
 Counterfeit Drugs In America: Crimes, Victims & Solutions: A primer about the threat counterfeit medicines pose to Americans.
Counterfeit Drugs In America: Crimes, Victims & Solutions: A primer about the threat counterfeit medicines pose to Americans.
 Former FBI Director Louis Freeh's 2017 report about the dangerous effects of drug importation.
Former FBI Director Louis Freeh's 2017 report about the dangerous effects of drug importation.
 Rogues Gallery Comics: An Illustrated Guide To Counterfeit Drug Crime: real-life stories of fake drug criminals and their cases.
Rogues Gallery Comics: An Illustrated Guide To Counterfeit Drug Crime: real-life stories of fake drug criminals and their cases.
Major Supply Chain Breaks:
FDA:
- Drug Supply Chain News
- Letters to Doctors


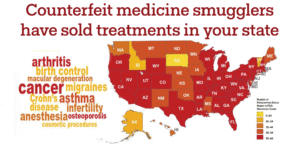 Read about fake medicine in your state
Read about fake medicine in your state