Update: In May 2017, Deanna Roberts pleaded guilty to illegally injecting persons with liquid silicone and for introducing liquid silicone that was obtained by fraud into interstate commerce. Roberts, who continued to administer industrial grade silicone injections even after she knew that one her patients had died and others had been hospitalized, was sentenced to…
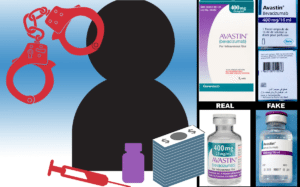
Timeline Leading to the Extradition of CanadaDrugs Principals Image courtesy of Legitscript.com According to CBC News, new information has come to light in the case against six executives of CanadaDrugs.com. The company, its affiliates, and associates in the United Kingdom and Barbados stand accused of illegally importing and selling $78 million worth of unapproved, misbranded…
June 29, 2017 This is a reprint of an FDA Alert. When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or the company. For Immediate Release Contact Consumers sales@hardcoreformulations.com 1-855-773-6826 San Marcos, TX, Hardcore Formulations is…
Email spam is one of the curses of our online lives. At the very least, it is a nuisance, and at its worst, it distributes false information, dangerous malware, and phishing messages. TG Daily recently reported how one company determined that there was not an online Canadian pharmacy behind email spam messages, but a massive…
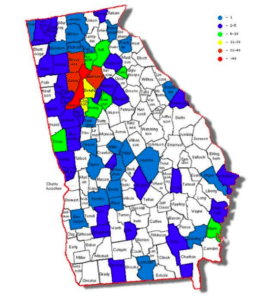
As reported by WSB-TV2 Atlanta, chemistry experts at the Georgia Bureau of Investigation (GBI) issued a public safety warning to the state’s citizens. Agents warned that the latest crisis is counterfeit pills containing fentanyl and fentanyl analogues sold to unsuspecting people on the street. GBI spokeswoman Nelly Mile called the situation unprecedented, noting that, “If…
Many people struggle to lose weight, and under a doctor’s supervision, diet pills can be a safe and effective way to reach your goal. Some people, like Elna Baker, are so afraid of regaining the weight that they continue taking the pills by purchasing them while in other countries or ordering them from online pharmacies. While…
Dr. Kenneth D. Nahum, his oncology practice, and Ann Walsh, his wife and the practice manager, have agreed to pay the U.S. $1.7 million to resolve allegations of violating the False Claims Act. They stood accused of ordering drugs from a foreign distributor not approved to sell them in the U.S. and administering those drugs…
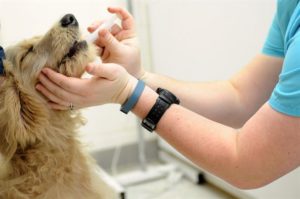
When Dr. Watts’ veterinary patient stopped responding to medication, Dr. Watts questioned the source of the medication. The pet’s owner purchased the medication from online. When the doctor called the manufacturer with the owner in the room, the manufacturer stated that they could not guarantee the product from that source and considered it potentially counterfeit.…
Online pharmacy Canada Drugs, under indictment for selling millions of dollars worth of fake and misbranded cancer medications to U.S. medical offices, still retains its license to operate from the College of Pharmacists of Manitoba, according to CBC’s Karen Pauls. Despite accusations from U.S. federal prosecutors for allegedly selling $78 million of unapproved, misbranded and…
Police in Ottawa made a disturbing discovery when conducting a drug bust, CBC Canada is reporting. In addition to finding a typical array of cash, drugs and guns, Ottawa police found a quantity of an “unknown powder” that has yet to be analyzed, and a counterfeit pill press capable of producing 20,000 counterfeit pills an…