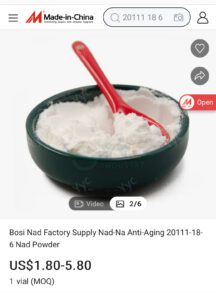
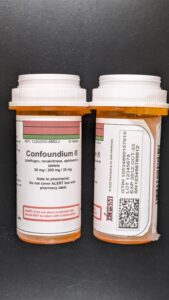
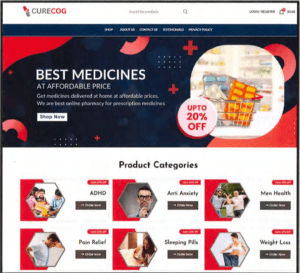
This week the agency warned about compounding safety and sterility issues and a newly posted document shows inspectors enforcing manufacturing standards.
Chinese companies and individuals indicted by the Department of Justice and a study examines how patients were harmed by the sudden removal of a medication from a drug formulary.
The FDA recently announced that “connected trading partners” that have made progress in transmitting electronic data that identifies and follow medicines made for the U.S. drug supply will not be penalized if they are still working out challenges in the process. Are you wondering what that means? We can explain.
Family advocates petition the Office of U.S. Trade Representative to take trade action over Chinese fentanyl precursors. The New York Times tackles PBMs and pharmacy deserts.
Consumers taking this product should immediately consult with their health care professional to safely discontinue use of this product. The risks of withdrawal from corticosteroids should be assessed by a healthcare professional. Only licensed health care professionals can evaluate patients for the risk, or confirm the existence, of adrenal suppression. Consumers that have product which is being recalled should return to place of purchase or discard.
The FDA granted exemptions for certain trading partners and small dispensers to avoid supply chain disruptions. Stories from the week in counterfeit medicine news.
Our Executive Director, Shabbir Safdar, along with PSM members, shared examples of pharmaceutical counterfeits and helped attendees understand why current systems for stopping counterfeit product sales do not work well.
An indictment by the Department of Justice against 18 individuals who allegedly sold counterfeit pills via dozens of fake pharmacy websites to residents in all 50 states prompts warnings from two additional federal agencies.
Twenty-seven people in Puerto Rico indicted in misbranded and diverted drug case, CBP seized counterfeit and misbranded drugs in two states, and fake arthritis and cancer medications seized in India.
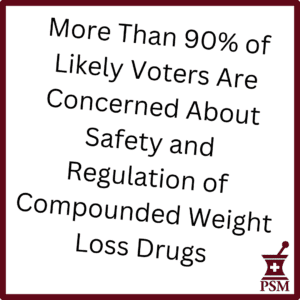
PSM commissioned a survey about compounded diabetes and weight loss injectable drugs. 1,000 likely voters across political affiliations were crystal clear — they are worried about safety and the current lack of oversight. See the full results.