FDA’s shutdown of GLP-1 compounding could lead to a new era of risk for U.S. patients.
Read MoreThe FDA published an update to a previous alert about undeclared active pharmaceutical ingredients in a dietary supplement to expand the package styles affected by this warning.
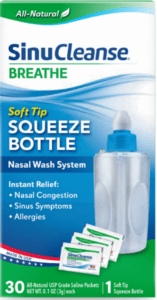
Read MoreAn FDA alert shared information on a nationwide recall of a nasal wash system due to microbrial contamination.
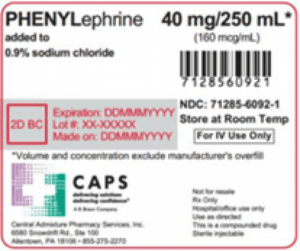
Read MoreAn FDA alert warned that Central Admixture Pharmacy Services issued a nationwide recall of Phenylephrine 40 mg added to 0.9% Sodium Chloride 250 mL in 250 mL Excel Bag due to the detection of black particulate matter in a single sealed vial of Phenylephrine Hydrochloride.
Read MoreAn FDA alert warned that Vitafer-L has been found to contain undeclared tadalafil, an active pharmaceutical ingredient. Products containing tadalafil cannot be marketed as dietary supplements.
Read MoreThe U.S. Attorney’s Office in the Western District of Washington has charged two Indian nationals with selling Americans counterfeit Keytruda.
Read MoreThere has been controversy about the ad Hims & Hers ran during the Super Bowl. And now an SEC filing says investors should be warned about risk if their ads are found to be non-compliant.
Read MoreThis is a reprint of an FDA Alert.
Read MorePSM’s new report shows that unregulated semaglutide and tirzepatide are slipping by FDA and CBP at the border. Semaglutide is no longer in shortage.
Read MoreThe Partnership for Safe Medicines today released a new report that found suspicious, unauthorized, and illegal ingredients for popular diabetes and obesity injectables (commonly known as weight loss drugs) are flooding into the U.S. from foreign sources despite U.S. laws forbidding them from coming through the border.
Read More