With seizures of counterfeit prescription pills made with fentanyl and other dangerous substances up 500% since 2019, a bipartisan group of Senators and Representatives have introduced S. 4151/H.R. 8175, the Stop Pills That Kill Act, to help law enforcement stop the deadly flow of these pills into our communities.
Read MoreThe NABP’s new report explores locking and suspending websites as a tool to stop fake medicines. Tainted children’s medicine is also in Indonesia. Mexican authorities find more counterfeit drugs. Britain sentences a wholesaler who sold stolen treatments. Deaths, prosecutions and hundreds of thousands of fake prescription pills in 19 states
Read MoreWatch this page for updates about litigation that families of victims filed in October 2022.
Read MoreFamilies sue Snapchat for facilitating the sale of fake pills. Authorities in Michigan charge two men with importing controlled medicines. The LA County Sheriff’s Department seized thousands of illicit pharmaceuticals. Prosecutions, seizures and deaths related to counterfeit prescription pills in 16 states.
Read MoreUse of these tests may cause serious adverse health consequences or death. There is also a risk of injury if users follow any labeling instructions directing self-collection of nasopharyngeal or oropharyngeal samples.
Read MoreAn Indian man admits to selling Americans smuggled, controlled medicines prescription-free. The U.S. Adderall shortage continues. Dangerous counterfeit medicines were found in Gambia, Mexico and Pakistan. Captagon being produced in Syria. News about counterfeit pills in 14 states and the District of Columbia.
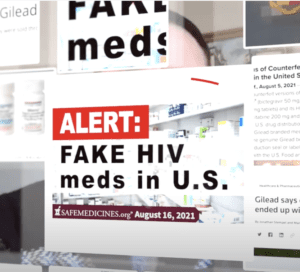
Read MoreA lawsuit over fake HIV drugs continues to expand. Syracuse University warned students about fake COVID teats. The DEA seized over ten million fentanyl pills in three months. Congress introduced a bill that would shift COVID-19 funds to fentanyl education and prevention in schools. Counterfeit pills stories in 16 states.
Read MoreProper Trade LLC/My Stellar Lifestyle was notified by Amazon that laboratory analysis has found the product to be tainted with tadalafil, an ingredient in FDA approved products for treatment of male erectile dysfunction in the family of drugs known as phosphodiesterase (PDE-5) inhibitors.
Read MoreBest Practices to Rid Social Media of Drug Trafficking will be released tomorrow at 10am Pacific. Sign up to watch in this post.
Xylazine in the illicit drug supply. Over 2.4 million counterfeit pills made with fentanyl seized across the country. Another 24 stories in 17 states.
Read MoreA parent-led commission will release recommendations for social media companies to curb drug dealers on their platforms on September 27th at 1pm Eastern / 10am Pacific.
Register for the launch event.
Read More