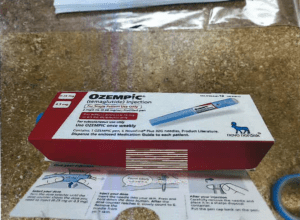
The Partnership for Safe Medicines applauds the Arkansas State Board of Pharmacy for utilizing new product verification technology for identifying illegitimate Ozempic quarantined by a pharmacy this week.
Read MoreMystery cosmetic injections allegedly harmed a client in Queens. PSM addressed a misleading drug ad for compounded weight loss medicine.
Read MoreYou could tell an American that they’re getting a compounded medicine and they would have no idea what that meant. That may be a legal disclosure, but that’s not good enough to protect patients who don’t know what the words mean.
Read MorePSM sent the following letter to the FDA’s Office of Prescription Drug Promotion (CDER) asking them to enforce the laws and guidelines that protect Americans from misleading advertising in the Hims&Hers Super Bowl ad.
Read MorePSM sent the attached letter to Fox Broadcasting, calling on them to withdraw the deeply troubling and misleading Hims&Hers Super Bowl ad.
Read MoreEmployees of the now-closed Chinese chemical company Hubei Amarvel Biotech were convicted at trial after shipping more than 200 kilograms of fentanyl precursor chemicals to the U.S. between November 2022 and June 2023.
Read MorePartnership for Safe Medicines released the following statement in response to the advertisement that lifestyle brand Hims & Hers released ahead of the Super Bowl to sell unregulated compounded weight loss drugs
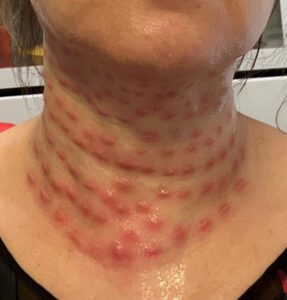
Read MoreLast week a Canadian man got 30 years for selling Americans counterfeit Xanax on the dark web and a New York spa owner was arrested after he allegedly injected patients with fake Botox
Read MoreA new FTC report examines how PBM business practices have inflated drug costs.
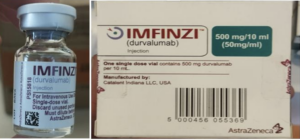
Read MoreInformation provided to WHO by AstraZeneca, the genuine manufacturer of IMFINZI, has confirmed that the products identified in this Alert are falsified. Laboratory analysis of samples of the falsified IMFINZI have been carried out by AstraZeneca. The analysis confirmed that the vials of the falsified product contained no active pharmaceutical ingredient.
Read More