In PSM’s round-up this week: FDA and FTC warnings over fake COVID-19 treatments and fraud, as well as fake medicine news in seven U.S. states, Canada and Cambodia.
Read MoreSeeking a good night’s sleep, 25-year-old Jake Beddoe, a young travel consultant with an adventurous heart and a tremendous sense of humor, took part of what he thought was a Xanax pill on May 27, 2020. The pill was counterfeit, and Jake died of fentanyl poisoning.
Read MoreIn PSM’s round-up this week: news about state drug importation, COVID-19 fraud, fake hand sanitizer stations, and more counterfeit medicine news.
Read MoreThis editorial by Brandon Macsata was published in The International Business Times on November 1, 2020. Macsata has been living with HIV since 2002, and serves as the CEO of the ADAP Advocacy Association, an organization that promotes the AIDS Drug Assistance Program and works to improve access to care.
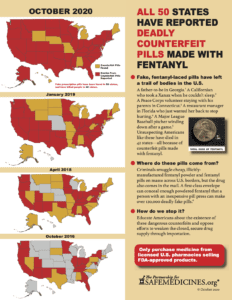
Read MoreWith the recent report that police in Maui, Hawaii seized 400 counterfeit oxycodone pills made with fentanyl on October 2, 2020, the United States has reached a sobering milestone: public sources have reported about fake pills made with fentanyl in all 50 states.
Read MoreIn PSM’s round-up this week: news about state drug importation, COVID-19 fraud, deadly workout supplements and counterfeit pills made with fentanyl.
Read MoreA summary briefing of 2020’s major cases, worrying trends and a deep dive into drug supply chain policy issues.
Read MoreIn PSM’s round-up this week: new reports about counterfeit medicine, drug supply chain regulation and phishing scams; FDA warnings about dangerous supplements; a life sentence for fake pill kingpin Aaron Shamo; and more.
Read MoreIn this analysis, which was published in Lexology on October 13, 2020, three global regulatory experts examine barriers to drug importation.
Read MoreIn PSM’s round-up this week: Policy truths about drug importation, prosecutions for fake COVID-19 treatments and financial fraud, and another week in counterfeit pills.
Read More