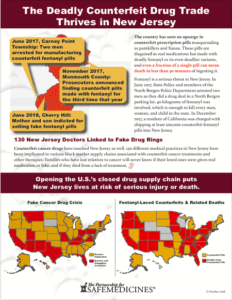
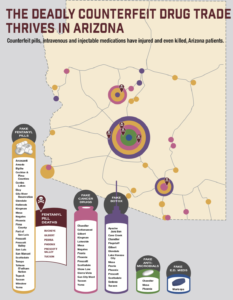
The Governor of New Jersey has just signed A-5037, a new law designed to increase penalties for counterfeit drug crimes committed in the state.
Read MoreThe U.S. Department of Justice announced that Stephan Caamano, who manufactured and sold at least 4.3 million counterfeit Xanax pills, will spend the next 13 years in federal prison after pleading guilty to his crimes…
Read MoreThis editorial by David C. Rosenbaum and Dara Jospé was published in the Financial Post on January 16, 2020. Rosenbaum is a partner of the law firm Fasken. Jospé is an associate for the same company.
Read MoreThe Maricopa County Medical Examiner has blamed fentanyl poisoning for the death of a four-year-old girl in Glendale last September. The report cited fentanyl toxicity as the cause of the little girl’s death.
Read MoreThis editorial by Dr. Kristina M. L. Acri née Lybecker was published in IP Watchdog on January 2, 2020. Dr. Acri is an Associate Professor of Economics at Colorado College in Colorado Springs, and Chair of the Department of Economics and Business.
Read MoreDr. Thomas Whalen, a rheumatologist who practiced medicine in Havertown, Pennsylvania has pleaded guilty to charges he purchased non-FDA approved, temperature-sensitive biologic medication from Turkey and the United Kingdom to treat his rheumatology patients.
Read MoreAuthorities seized close to 40,000 fake Adderall pills from Rodriguez-Rabin’s apartment. There was also a “machine to make the pills” seized during the raid. Rodriguez-Rabin is a 51-year-old lecturer that worked in the University of Texas system.
Read MorePartnership for Safe Medicines Statement on Proposed Regulations to Import Prescription Medicines from Canada Washington, D.C. (December 18, 2019) – Shabbir Safdar, executive director of the Partnership for Safe Medicines, released the following statement in response to today’s announcement by the Trump Administration and the proposed regulations to allow importation of prescription medicines: “Citizens of…
Read MoreConsumption of a product with undeclared tadalafil may pose a risk to consumers who take prescription medications containing nitrates (such as nitroglycerin). The combination of tadalafil and nitrates may lower blood pressure to dangerous levels which can be life threatening. Consumers with diabetes, high blood pressure, high cholesterol, or heart disease often take nitrates and may be the population most likely to be affected.
Read MoreThis editorial by Sally Pipes was published in The Sun Journal on December 16, 2019. Pipes is president, CEO and Thomas W. Smith Fellow in Health Care Policy at the Pacific Research Institute.
Read More