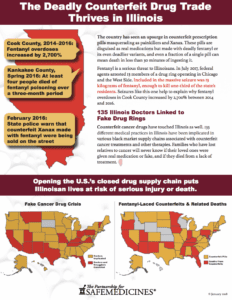
On March 21, 2019, 22-year-old Jacob Reis of Cary, Illinois pleaded guilty to charges he gave his 19-year-old girlfriend, Rachel Ramirez, a deadly fentanyl-laced counterfeit pill that killed her, the Northwest Herald reports. According to a 2018 article in the Northwest Herald, Reis and another young woman, Reanna Salas, were originally charged with providing the…
This editorial by Guy Anthony was published in the Orlando Sentinel on June 12, 2019. Anthony, President and CEO of Black, Gifted & Whole, a nonprofit focused on issues surrounding black, queer men, warns that drug importation will open up “a market for dangerous, counterfeit drugs” that will make it harder for people to live with HIV and other complex illnesses.
We’ve all heard politicians promise cheaper drugs imported from other countries, but are they just saying that because we want to hear it? Do they have any expertise that suggests they know how to make such a system safe?
This editorial by Nigel Rawson was published in The Hills Times on June 9, 2019. Dr. Rawson, president of Eastlake Research Group, a senior fellow at the Fraser Institute, and an affiliated scholar with the Canadian Health Policy Institute, warns that Canada would run out of necessary medicine if U.S. states begin drug importation programs…
Washington, D.C. (June 11, 2019) – Shabbir Imber Safdar, executive director of the Partnership for Safe Medicines, released the following statement today in response to the signing of Florida’s drug importation bill: “Today, Florida’s governor put politics over the safety of residents across the state. Although this was widely expected, we remain committed to protecting…
Three Los Angeles-based companies, and five individual defendants have proffered guilty pleas on charges that they were making and distributing herbal supplements containing dangerous levels of prescription pharmaceuticals.
“I’m pleased to re-join the Partnership for Safe Medicines’ Board of Directors at a time when factual information concerning the risks posed by medicines reaching unsuspecting Americans is critically needed” said Tom.
Former DEA agent Doug Herber wrote this editorial, which was published on May 31, 2019 in the White Mountain Independent. In it, he writes that drug importation will cause “patients [to] unwittingly purchase foreign counterfeit drugs disguised as low-level medication, unaware of the dangers, end up as an overdose statistic. “
According to the Department of the Treasury’s Under Secretary for Terrorism and Financial Intelligence, Sigal Mandelker, “The Goldpharma network illustrates the sophisticated tactics drug traffickers and money launderers use to capitalize on the Internet and online pharmacy sites to sell highly addictive illicit narcotics around the world.”
This editorial by Holly Strom and Kenneth Schell published in U.S. News and World Report on May 28, 2019 warns states considering drug importation that doing so will not keep costs down and also poses a safety risk to patients…