Exposes in the Houston Chronicle and Endpoints News examined Empower Pharmacy’s safety record. A grand jury indicted a Chinese company and three of its employees for pill press and counterfeit die mold sales.
The FDA wants to ensure that domestic and foreign companies receive the same level of regulatory oversight.
Catch up on research PSM released about medicines imported from unregistered sources.
What if your blood thinners were made in an unverified location in Colombia? In March 2025, dozens of pharmaceutical shipments entered the U.S. that were manufactured in places no one would expect.
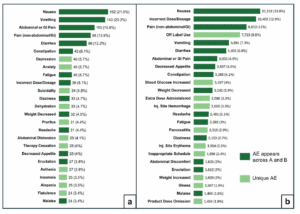
New research shows a correlation between compounded weight loss and diabetes injectables and increased adverse events. GIlead Sciences is suing another New York City pharmacy that allegedly sold counterfeit HIV medicine.
The FDA has issued a warning to the operators of the online pharmacy www.thesafepills.org for illegally selling unapproved and misbranded opioid medications, including tapentadol products marketed as Aspadol and Typendol. These unauthorized drugs, sold without a prescription, pose significant health risks such as overdose, addiction, and even death.
One of SafeChain’s three owners pleaded guilty. The company collected secondhand HIV medicines from patients and resold them to licensed U.S. pharmacies, forging paperwork to make them look legitimate.
A Kentucky doctor treated weight loss patients with research chemicals; CBP seized 90,000 alprazolam pills being smuggled into the U.S.
The FDA and Novo Nordisk are warning the public about counterfeit Ozempic injections circulating in the U.S. drug supply chain. The falsified products, labeled with lot number PAR0362 and serial numbers beginning with 51746517, were seized by the FDA on April 9, 2025. Their contents and safety are unverified and pose serious health risks.
PSM is seeking input on developing a set of best practices to reduce sales of counterfeit and diverted medicines on online pharmacy-to-pharmacy marketplaces.
The FDA announced that it had seized counterfeit Ozempic injections on April 9. PSM testified at a congressional hearing.