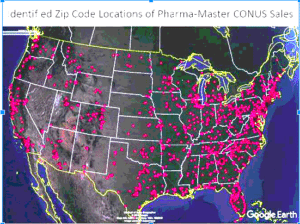
Police in Northfield, Minnesota warn of counterfeit oxycodone pills made with carfentanil in their city. These pills already caused at least three non-fatal poisonings…
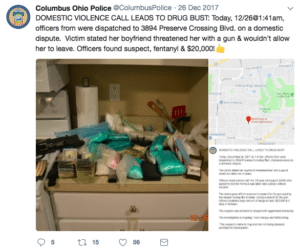
Ohio resident Alexander Henize faces 16 counts after a search of the home he shared with his girlfriend and their 2-year-old daughter turned up an industrial pill press and 11 pounds of fentanyl…
FDA Commissioner Scott Gottlieb recently toured the International Mail Facility at JFK Airport with members of Congress so they could see firsthand what the FDA is attempting to sort through to keep dangerous opioids out of the country and why they need a staffing increase…
The Mexican Federal Police recently stopped a vehicle enroute to California carrying over 100 pounds of fentanyl.
Other countries, even advanced countries like Canada, don’t provide the same level of protection. From April 2016 to March 2017, Canadian agents discovered more than 5,500 packages of counterfeit drugs in their midst. Loosening importation restrictions would expose American patients to potentially deadly counterfeit pills.
A Phoenix area man has been sentenced to 3 years in prison for transporting thousands of fentanyl-laced pills into Arizona.
Netflix DEA NARCOS agents Javier Pena (DEA Special Agent (Ret.) and Steve Murphy (DEA Special Agent (Ret.) describe the growing nexus between illicit drug traffickers and counterfeit medicines.
Short updates on two major fentanyl counterfeit pill ring cases. One case in Texas looks to have one of the fourteen defendants taking a plea while the second case in Utah sees a jury trial date set for Aaron Shamo, the alleged drug ring leader that shipped counterfeit pills containing fentanyl all across the U.S…
Most people in the U.S. live in states where their pharmacists are barred from letting them know that they could save money by paying cash instead of their insurance’s co-payment. Federal and state legislators are looking to change that…
U.S. Department of Justice announced two new cases. One is against a PA couple for manufacturing and selling counterfeit prescription pills made with heroin. The second case is against an OH couple charged with running a drug ring that imported and sold fentanyl and carfentanil in the Akron area…