Drug Importation in New Hampshire: An Overview
Current status:
New Hampshire submitted an application for its state importation program in April 2021. The FDA rejected the application in November 2022 because it did not identify a Canadian wholesaler that would provide the drugs.
Synopsis:
In January 2020, the New Hampshire legislature introduced SB685, which would require the New Hampshire Department of Health and Human Services to design a wholesale importation program for prescription drugs from Canada by or on behalf of the state and obtain federal approval for the program. In June 2020, SB685 was added to HB1280, which included several other prescription drug initiatives. The bill was signed into law in July 2020.
How should we evaluate this program?
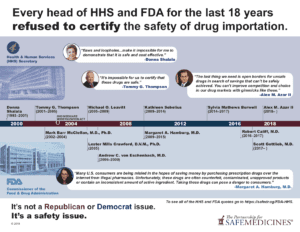
The program hasn't started yet, or even been designed, so there's no way to measure whether it saved money or kept patients safe, both promises made at the time of passage. However, the 2003 Medicare Modernization Act contains requirements for safety requirements built into any such program.
Official actions and statements
November 2022: FDA denies the application.
April 2021: New Hampshire submits an SIP application.
HB1280: Text of the Bill
SB685: Text of the Bill | New Hampshire State Senate hearing
Testimony opposing SB685:
- The Best Medicines Coalition, January 21, 2020
- The Partnership for Safe Medicines, January 2020
Background / resources
Learn more about
Op-eds from the Experts
Former DEA agent Doug Herber wrote this editorial, which was published on May 31, 2019 in the White Mountain Independent. In it, he writes that drug importation will cause “patients [to] unwittingly purchase foreign counterfeit drugs disguised as low-level medication, unaware of the dangers, end up as an overdose statistic. “
This editorial by Holly Strom and Kenneth Schell published in U.S. News and World Report on May 28, 2019 warns states considering drug importation that doing so will not keep costs down and also poses a safety risk to patients…
In this editorial, which was published in The Bend Bulletin on May 21, 2019, Canadian law enforcement veteran Don Bell explains why Oregonians can not rely on Canada for safe prescription drugs.
Dozens of the most highly qualified law enforcement officials and former, senior staff at the U.S. Food & Drug Administration have conducted in-depth analyses that show Canadian drug importation will lead to a massive increase in counterfeit drugs entering the U.S.
In this editorial published in The Hill on May 12, 2019, Brooklyn Roberts, the director of the health and human services task force at the American Legislative Exchange Council, discusses the risks of drug importation:
“The safety of our prescription drugs relies on a closed system where drugs can be traced to manufacturers, distributors, pharmacies and patients. Opening that system to foreign drugs would allow the potential for dangerous and potentially deadly medicines to land in the hands of the American public.”
In this editorial published on April 30, 2019, former FBI Director Louis Freeh talks about the safety risks of drug importation: “There are hidden risks and costs associated with the scheme that have not been getting much attention which impact your health and Colorado law enforcement’s ability to keep us safe.”
In this editorial published on April 26, 2019, Douglas Holtz-Eakin, economist and President of American Action Forum, questions economic truths about drug importation:
“Drug reimportation has long been the fool’s gold of health policy, and the Florida bill is no different. It flunks a basic policy analysis. But most amazing, it is drafted to raise hope, but not actually help Floridians.”
In this piece published in the Washington Free Beacon on April 25, 2019, staff writer Charles Fain Lehman explores issues around Florida’s drug importation proposal. “Critics,” he notes, “fear that the actual realities of regulatory oversight—especially in the hand of an as-yet-unnamed private vendor—will simply be too challenging to manage responsibly.”
In this piece, which was published in The Deseret News on April 24, 2019, pharmacist and Utah State Senate Majority Leader Evan Vickers raises serious concerns about importation as a strategy to lower drug prices:
“Anyone who truly understands how drugs are sold and distributed in the U.S. knows that there are very solid technical reasons that such importation is not viable. There are also serious concerns about drug safety, since the CHS cannot guarantee origin and purity on foreign-sourced drugs.”
In this piece, which was published on the ABC affiliate WJLA’s website on April 25, 2019, political analyst Boris Epshteyn explains that “this is a risky plan that will make it difficult to ensure that Floridians are receiving real and safe medicine.”