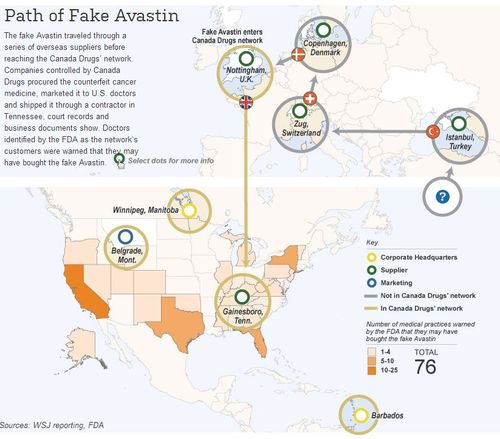
This graphic from the Wall Street Journal shows the path of the fake Avastin as it was shipped and reshipped around Europe to disguise it's true pedigree. Such shipping paths help make fake medication look more real because it comes from a "safe" country.
Why doesn't it work to import medicine from Canadian pharmacies or wholesalers for American use?
Importing medicine from Canada doesn't work for a number of reasons, not the least of which is that Canada is a country of 37 million people struggling with drug shortages today, and America is a country of 300+ million people that would empty the shelves of Canadian pharmacies in less than seven months if we started buying.
More importantly though, there's a long history of criminal Canadian drug sellers, some with very real licenses, preying on Americans. Often the only thing Canadian about their medicine is the maple leaf flag on their website.
Addressing the cost of healthcare is an urgent priority for many Americans, and PSM urges policymakers to address it. There were over 75 bills in the last U.S. Senate filed to address the cost of medication, and many more on the overall cost of healthcare. But it's never ok to sacrifice safety to achieve savings.
Below you can learn more about why importation doesn't work.
Top ten reasons it doesn't work to import medicine from Canada
#10: Canadians are not inspecting medicine bound for the United States.
Today a great deal of the medicine that Americans think they are getting from Canada is actually transshipped through Canada. It comes from third world black market manufacturers in countries like China, Turkey, or Pakistan, and arrives at an international mail facility in major cities such as Toronto. Instead of passing through Canadian customs, it is reshipped to America. Canadian authorities have been explicit that they will not inspect these packages. The FDA has documented the use of transshipping through Canada as source of counterfeit medication in America.
#9: It breaks Track and Trace.
Every American proposal to import medicines from Canada insists that it will be a part of the Drug Secure Supply Chain Act implementation called “Track and Trace,” but the only people who say that are people don’t know how Track and Trace actually works. We are five years and hundreds of millions of dollars into a ten-year implementation of Track and Trace and it does not include Canadian pharmacies or wholesalers. Nor does Canada have a reciprocal Track and Trace program.
Insisting that these medicines will be protected by Track and Trace is a policy promise that cannot be fulfilled.
#8: Counterfeit medicines rob patients of their only chance at treatment.
Patients dealing with a serious illness are already racing the clock to arrest the progress of their disease. If the unapproved medication they are taking proves to be counterfeit, their disease will progress unchecked. A sub-therapeutic or outright placebo treatment for a patient with cancer, for example, will allow that cancer to spread, potentially losing the window for survival.
An HIV patient who inadvertently takes a drug cocktail, with a sub-therapeutic dose of one medicine may find that their strain of HIV has become permanently resistant to the entire cocktail. When this happens, their treatment options narrow in a way that can threaten their life.
#7: We’re already struggling to keep rogue medicines out of our drug supply.
In the past ten years many criminals, including some with Canadian pharmaceutical retail and wholesale licenses, have been caught selling fake medicines to American patients and medical clinics. Five different criminal rings (Gallant, CanadaDrugs.com, Ozay Pharmaceuticals, TC Medical and Medical Device King) successfully did business with medical practices in almost every state in the U.S. We still don’t know how many thousands of American patients were harmed and will probably never know the full scope.
#6: It’s difficult to regulate people and businesses that don’t fear prosecution.
One of the biggest flaws with states licensing foreign pharmaceutical wholesalers and pharmacies is that states cannot discipline them as they would businesses in their own state. The foreign actors have no assets and no physical presence in the state proposing importation. The state board of pharmacy cannot enforce an order to a foreign pharmacy to stop selling spoiled product, shut dangerous facilities, or suspend workers with poor safety records.
When the Justice Department indicted Canadian pharmacist and CEO Kris Thorkelson in a scheme involving counterfeit cancer medication, he simply refused to come to the U.S. for trial. Extradition proved impossible, and in 2018 the U.S. gave up and agreed to a plea bargain that involved no jail time for him.
#5: Importation has been tried in seven states and failed to save money, burned state funds, and endangered patients.
IL, MO, KS, VT, WI, MN, and ME have all attempted to create importation schemes in the last fifteen years. None of these programs are still in existence now. All of them had documented patient endangerment issues that made them less safe than the existing drug supply chain. Furthermore, when the first six shut down they all had very low utilization due to the fact that changing insurance coverage and the power of the generics market had eliminated their cost savings. In Maine, the program was shut down by a federal judge.
#4: Importation will worsen the opioid crisis and endanger law enforcement.
Every state in the U.S. is also facing a new threat: counterfeit pills made with fentanyl. These pills are killing Americans who don’t realize the dangers posed by counterfeit medicines precisely because our drug supply has been so safe.
The fentanyl used to make these pills also poses a danger to first responders and—because it is so potent that it can be shipped in tiny quantities—makes their efforts to intercept it much more difficult. This is why drug importation is opposed by the National Sheriffs Association, the Major County Sheriffs Association, the International Association of Chiefs of Police, National Association of Drug Diversion Investigators, among others.
#3: Importation is opposed by healthcare professionals and regulators on both sides of the border.
Importation is opposed by: boards of pharmacy in both Canada and the US, both the American and Canadian Pharmacist Associations, both the American and Canadian pharmaceutical wholesalers association, by several Canadian health ministers, by every FDA commissioner and HHS secretary since 2000, by American patient groups concerned they will get subtherapeutic medication, and by Canadian patient groups concerned that importation will create even more shortages and create price spikes in Canada.
#2: Canadian patients don’t want it.
There are currently over 1,700 drugs in shortage in Canada. Canada also has the second highest drug prices in the world. For this reason Canadian patient groups have opposed American importation schemes. They see increased shortages and price spikes as the consequence of allowing Americans to plunder their medication.
#1: 37 million Canadians cannot supply 300+ million Americans with medicine.
On its face, the size difference between the countries should tell you that importation is a non-starter. Canada will not willingly allow America to raid its approved drug supply at the expense of Canadian patients, so the only available source of medicine will involve the black market. We should all be able to agree that importing black market drugs is not safe.
For more information on the structural flaws in foreign drug importation programs, please see: https://www.safemedicines.org/policymakers-media