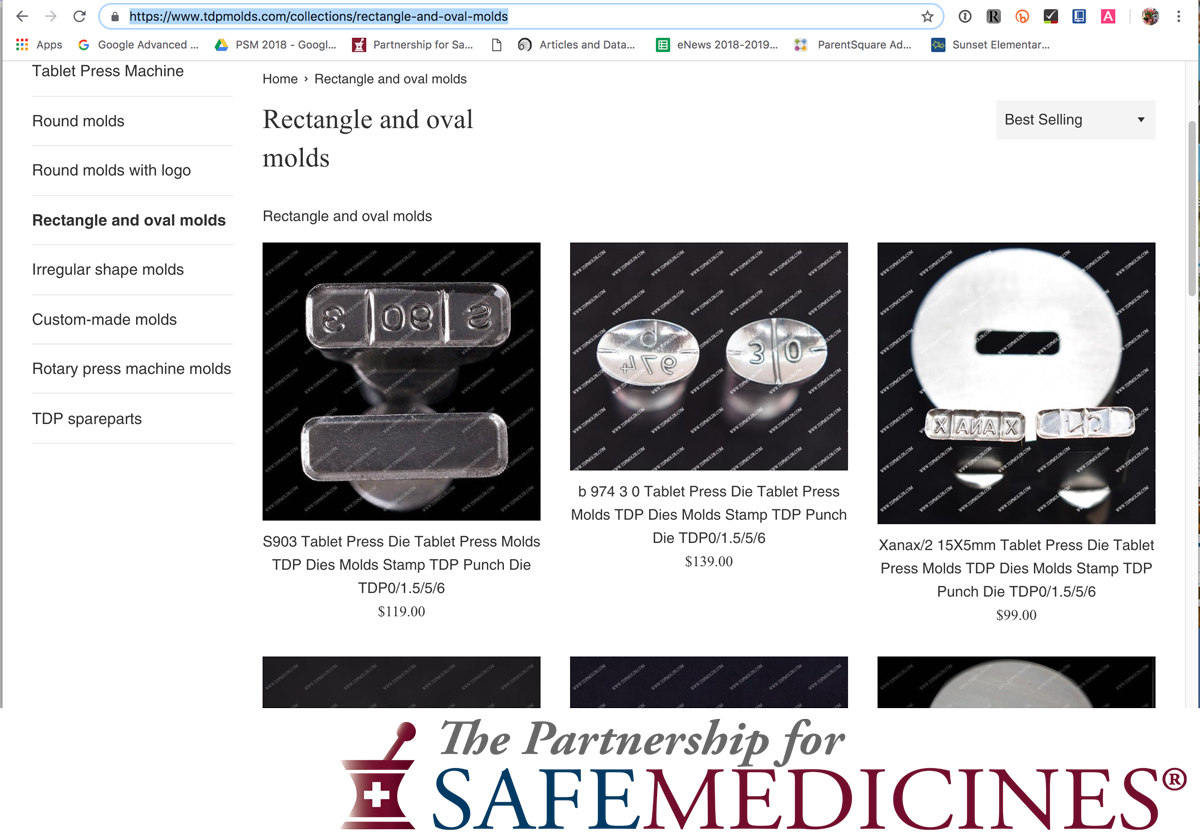
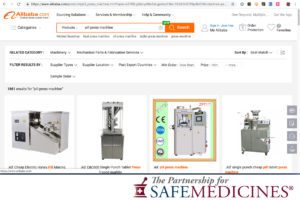
In April 2021, The National Association of Drug Diversion Investigators and The Partnership for Safe Medicines released an update to Illegal Pill Presses: An Overlooked Threat To American Patients.
Experts, Victims and Law Enforcement Officials to Join Virtual Roundtable Discussion on the Growing Threat Posed by Illegal Pill Presses
Former FBI Director Louis Freeh to Address Implications for Law Enforcement and Proposals to Legalize Drug Importation; New Study Highlights the Prevalence and Availability of Pill Presses
The supply and demand of dangerous counterfeit and illegally-imported medications has created one of our country’s most serious health challenges. Counterfeit pills made with fentanyl have been found in 46 states, and the prevalence of illegal pill presses are contributing to this crisis.
The National Association of Boards of Pharmacy, National Association of Drug Diversion Investigators, and The Partnership for Safe Medicines have collaborated on new research that analyzes the availability of pill presses, how these illegal devices enable criminals to manufacture counterfeit medicines, potential solutions to deter use, decrease risks, provide effective tools for law enforcement, and improve patient information and resources.
On Tuesday, March 19, 2019, a panel of experts and victims joined together to discuss the threat posed by pill presses and speak to the findings of the new study.
Additional report materials:
- Executive summary
- Full report: Illegal Pill Presses: An Overlooked Threat To American Patients
- Letter from families of counterfeit drug victims to elected officials, March 19, 2019
- Photographs from the report are available below.
Watch highlights from the March 19, 2019 webinar release of the new research/white paper (or watch the entire event on YouTube):
Speakers:
- Moderator: Samuel J. Louis, Member, Clark Hill and Strasburger as part of the Healthcare, Food and Drug Law and White Collar practice groups, former Assistant United States Attorney for the Southern District of Texas
- Louis Freeh, Former FBI Director
- George Karavetsos, former director of the U.S. Food and Drug Administration Office of Criminal Investigations and partner with the global law firm DLA Piper
- Shabbir Safdar, executive director of the Partnership for Safe Medicines
- Families who have been affected
- Gregg Jones, National Association of Boards of Pharmacy
Additional event materials:
- Video of the full event (YouTube)
- Presentation slides
- Video of a PSM staff member demonstrating the ease of using a $40 manual pill press:
Images from the report