News Coverage
The Partnership for Safe Medicines has been publishing information about the counterfeit drug problem around the world for more than a decade. With experts leading the organization and a committed and passionate set of writers and editors, our content is more in-depth than many other sources, which simply copy links to the news from other websites.
Last week a Canadian man got 30 years for selling Americans counterfeit Xanax on the dark web and a New York spa owner was arrested after he allegedly injected patients with fake Botox
A new FTC report examines how PBM business practices have inflated drug costs.
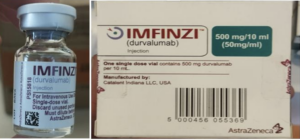
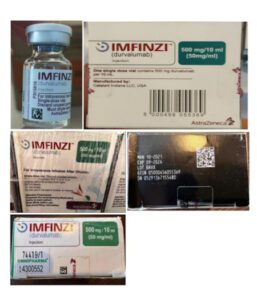
Information provided to WHO by AstraZeneca, the genuine manufacturer of IMFINZI, has confirmed that the products identified in this Alert are falsified. Laboratory analysis of samples of the falsified IMFINZI have been carried out by AstraZeneca. The analysis confirmed that the vials of the falsified product contained no active pharmaceutical ingredient.
WHO reported a fake cancer treatment. This year’s Notorious Markets report focused on fake online pharmacies. Board member Andrea Thomas urged fraternities to stock Narcan.
As prescription medications containing fentanyl and other illegal substances are becoming more widely accessible worldwide, a global crackdown on illicit drug precursors is leading cartels to experiment with the contents of their products.
FDA analysis has found these products to contain undeclared Furosemide, Dexamethasone and Chlorpheniramine. Furosemide was found at 5.24 mg/g or 1.84 mg/capsule. Dexamethasone was found at 2.22 mg/g or 0.780 mg/capsule. Chlorpheniramine was found at 4.38 mg/g or 1.54 mg/capsule.
Two new cases involving black market medicine in Texas and Tennessee, and CPB seized thousands of pills in Laredo.
The lawsuit against Snap may proceed and the ADA issued a statement about compounded GLP-1 medicines.
A California man must pay millions in restitution for selling fake HIV meds. Additional news about Virginia’s state drug importation plans, prosecutions around fentanyl pills, and overseas incidents.
A California man allegedly processed payments for foreign online pharmacies selling fentanyl and meth pills.
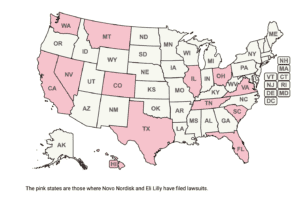
Novo Nordisk filed five more lawsuits against businesses selling compounded semaglutide. Dr. Tim Mackey spoke about the dangers of the unregulated semaglutide sales online, and South Africa warned residents about counterfeit Ozempic.
Consumption of products with undeclared sildenafil may interact with nitrates found in some prescription drugs (such as nitroglycerin) and may cause a significant drop in blood pressure that may be life threatening. People with diabetes, high blood pressure, high cholesterol, or heart disease often take nitrates. Among the adult male population, who are most likely to use this product, adult males who use nitrates for cardiac conditions are most at risk.
Use of products with undeclared diclofenac may cause increased risk of cardiovascular events, such as heart attack and stroke, as well as serious gastrointestinal damage, including bleeding, ulceration, and fatal perforation of the stomach and intestines. This hidden drug ingredient may also interact with other medications and significantly increase the risk of adverse events, particularly when consumers use multiple NSAID-containing products.
Another China-based chemical company was indicted for allegedly selling precursor chemicals and xylazine to U.S. buyers as Americans continue to grapple with the toll of counterfeit prescription pills made with dangerous drugs.
On August 14, 2024, FDA received a complaint from a patient who noticed a black particulate in a vial of semaglutide distributed by Fullerton Wellness. On September 23, 2024, FDA received information from California regulatory authorities as part of ongoing collaboration between FDA and the state noting deficiencies found at Fullerton Wellness during a state inspection. After the state inspection, Fullerton Wellness voluntarily ceased operations.
The agency urges manufacturers, including repackagers, to clearly identify any ingredients intended for use in foods or dietary supplements on the label. Providing this information on ingredient labels could help prevent compounders from using ingredients that are not appropriate for sterile drugs and may help prevent patient harm.
This week the agency warned about compounding safety and sterility issues and a newly posted document shows inspectors enforcing manufacturing standards.
Chinese companies and individuals indicted by the Department of Justice and a study examines how patients were harmed by the sudden removal of a medication from a drug formulary.
The FDA recently announced that “connected trading partners” that have made progress in transmitting electronic data that identifies and follow medicines made for the U.S. drug supply will not be penalized if they are still working out challenges in the process. Are you wondering what that means? We can explain.
Family advocates petition the Office of U.S. Trade Representative to take trade action over Chinese fentanyl precursors. The New York Times tackles PBMs and pharmacy deserts.
Consumers taking this product should immediately consult with their health care professional to safely discontinue use of this product. The risks of withdrawal from corticosteroids should be assessed by a healthcare professional. Only licensed health care professionals can evaluate patients for the risk, or confirm the existence, of adrenal suppression. Consumers that have product which is being recalled should return to place of purchase or discard.
The FDA granted exemptions for certain trading partners and small dispensers to avoid supply chain disruptions. Stories from the week in counterfeit medicine news.
Our Executive Director, Shabbir Safdar, along with PSM members, shared examples of pharmaceutical counterfeits and helped attendees understand why current systems for stopping counterfeit product sales do not work well.
An indictment by the Department of Justice against 18 individuals who allegedly sold counterfeit pills via dozens of fake pharmacy websites to residents in all 50 states prompts warnings from two additional federal agencies.
Twenty-seven people in Puerto Rico indicted in misbranded and diverted drug case, CBP seized counterfeit and misbranded drugs in two states, and fake arthritis and cancer medications seized in India.
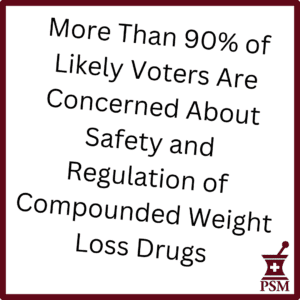
PSM commissioned a survey about compounded diabetes and weight loss injectable drugs. 1,000 likely voters across political affiliations were crystal clear — they are worried about safety and the current lack of oversight. See the full results.
The Federal Trade Commission issued an administrative complaint against the three largest pharmacy benefit managers, alleging that the companies used their market power to inflate the cost of insulin for U.S. patients.
If used chronically at the recommended dose, dexamethasone could cause severe and serious adverse events such as adrenal suppression (a disorder in which the adrenal glands do not produce enough hormones), central nervous system and psychiatric/behavioral effects, weight gain, gastrointestinal effects, elevated blood glucose, increased infection risks, neuromuscular and skeletal side effects, ocular effects, cardiovascular effects, dermatologic effects endocrine and metabolic issues, among other adverse events not mentioned.
Rxeed’s suit of CVS Caremark alleges predatory practices against independent pharmacies. Walgreens joins up with NABP’s Pulse. Stories about medicine safety in the U.S. and around the world.
Reuters writes about fake Ozempic and medicine tracking. The U.S. FDA issues warnings about supplements that contain undeclared prescription medicine. Additional news in India and Nigeria.
Guilty pleas in two federal cases involving dangerous medicines. Pill press news in South Carolina. Additional news in the U.K., India and Pakistan.
Researchers have found pentobarbital in counterfeit prescription pills. Plus, domestic and international news, and what our executive director is reading this week.
This is a reprint of an FDA Alert. Boulla LLC Issues Voluntary Nationwide Recall of Boom Max Capsules Due to the Potential Presence of Undeclared Sildenafil When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or…
A whistleblower complaint alleges that counterfeit and misbranded diabetes medical devices were being sold by third-party vendor on Amazon and orders were fulfilled by Amazon.com itself.
A new lawsuit says that another ring has been selling counterfeit HIV medicine in the U.S. Eli Lilly is taking action against compounders selling tirzepatide. More domestic and international news.
A New York couple admitted to selling sildenafil in supplements they claimed were “all-natural.” Ethiopian researchers say that one-fifth of medicines in African are fake or substandard. Additional news in Canada, the U.K, Thailand and Kurdistan.
A white paper examines how to detect and respond to substandard and fake medicine. A small study shows why buying semaglutide on line is dangerous. Police in Ukraine busted a counterfeit medicine drug ring. These stories and more in this week’s news.
A study published Friday in the Journal of the American Medical Association (JAMA) underscores the urgency.
The FDA warns that patients are harming themselves using compounded semaglutide. Cases involving pill presses in two U.S. states and two other countries.
FDA has received reports of adverse events, some requiring hospitalization, that may be related to overdoses due to dosing errors associated with compounded semaglutide injectable products.
A Michigan company is suing a telepharmacy that allegedly supplied fake Ozempic. New resources about the risks of compounded medicines. Details about the fake cancer drug ring busted in India.
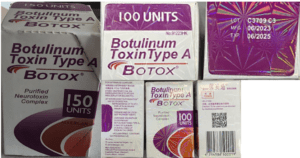
New information on the patients recently hurt by counterfeit Botox, two news stories involving pill presses, and a prosecution announced in Austria over fake Ozempic that sent multiple people to the hospital.
The Cooper Davis and Devin Norring Act would force social media to report online drug sales. Additional news involves counterfeit cancer drugs sold in the U.S., fake cosmetic injections and more.
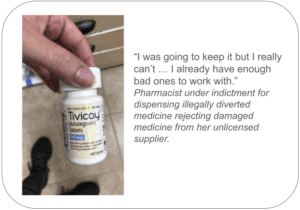
Adam Brosius, Patrick Boyd and Charles Boyd are facing eight counts for selling $90 million of diverted prescription drugs from five black-market suppliers.
On June 13, 2024, PSM sent a letter to Congressmen Darrell Issa and Jerrold Nadler applauding the reintroduction of the Stopping Harmful Offers on Platforms by Screening Against Fake E-Commerce (SHOP SAFE) Act.
Fake Ozempic has been found in Brazil, the U.K., and the U.S. Eli Lilly warned the public about fake and compounded tirzepatide medicines. Additional domestic and international stories.
Florida still has not imported a single pill, patients seeking GLP-1 medicines at increased risk from phishing scams, the counterfeit medicine news from three other countries.
“Lilly Warns Patients About Counterfeit and Compounded Medicines Releases Open Letter and Takes Further Legal Action Against Counterfeit, Fake, Unsafe, and Untested Products”
The FDA published its annual drug shortage report for 2023, CBP in Cincinnati seized multiple shipments of fake Ozempic, and international counterfeit medicine stories out of six different countries.
PSM submitted a letter to leaders in the New York State legislature opposing a legislative proposal (AB 7954) to setup a wholesale Canadian drug importation program. The bill died right before end of session.
PSM and ADAP Advocacy launched a campaign today about the dangers of medicine diversion criminals operating on gay dating apps such as Grindr. Learn more and report the crime on our campaign page.
The CAST Act introduced in the Senate. More lawsuits against copycat Wegovy sellers. Pill presses seized in multiple countries. Read about these and other stories in our weekly counterfeit medicine news roundup.
A Colorado board is considering setting a price limit on the medicine Stelara. This will have unintended consequences, undermining the safety of the supply chain for this medicine and limiting patient access. Learn more by reading our testimony.
Executive Director Shabbir Safdar’s editorial ran on May 15, 2024 in Colorado Politics
States like Colorado are experimenting with Prescription Drug Affordability Boards to address the price of medicine, but solutions like Upper Payment Limits will yield multiple unintended consequences that will do more harm than good to patients.
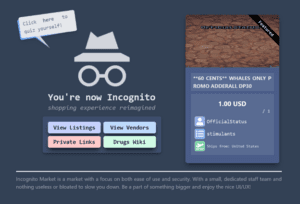
The operator of Incognito is charged with engaging in a continuing criminal enterprise, narcotics conspiracy, money laundering and conspiracy to sell adulterated and misbranded medication.
PSM is launching a public awareness campaign about the dangers of fake Botox. The campaign includes a dozen slides with quotes from a fake Botox victim about her physical effects. You can view the campaign elements in the gallery below or on PSM’s instagram channel as it rolls out over the next few weeks.
A counterfeit Botox victim described her illness to a reporter at Glamour. Additional news, including medicine sales on TikTok; a California man who allegedly sold imported etizolam; counterfeit, contaminated injections in India; and pill press seizures in three states.
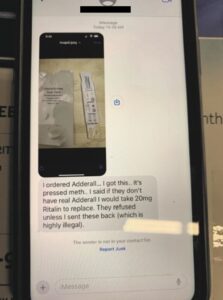
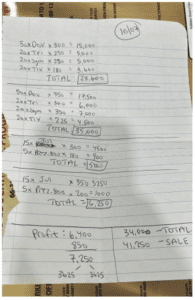
The indictment and pre-sentencing memo from the Southern District of New York’s prosecution of Boris Aminov offer a “how-did-they-do-it” guide to prescription drug health care fraud, capturing text messages, crime photos, and a step-by-step guide to the deceit.
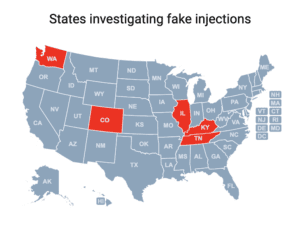
DEA seizures of fentanyl pills tripled between 2021 and 2023. The CDC narrowed the scope of the counterfeit Botox outbreak to nine states. Additional news about counterfeit medicine in seven states.
A New York woman has been charged with selling illegally imported weight loss drugs on TikTok. The U.S. Senate is considering legislation to ban tianeptine. A counterfeit Xanax vendor in Missouri was sentenced. More news in California, Colorado, Louisiana, and Texas.
After lengthy waits, men in two separate cases were extradited and have been sentenced for illegal sales of controlled medicines. Courts are still waiting on a Canadian man accused of selling 15 million fake Xanax pills to U.S. buyers. Biden signed the FEND Off Fentanyl Act. NABP released a report about illegal sales of GLP-1 agonists.
The NABP reports that illegal actors are selling desperate patients substandard and counterfeit versions of the new generation of weight loss medicine.
STOP CLOPEZ CORP is voluntarily recalling one lot of Schwinnng capsules to the consumer level. FDA analysis has found the Schwinnng products to contain Nortadalafil. Nortadalafil is an active drug ingredient known for the treatment of male erectile dysfunction. The presence of Nortadalafil in Schwinnng capsules makes it an unapproved new drug for which the safety and efficacy have not been established and, therefore subject to recall.
The Southern District of New York sentenced criminals from two separate HIV drug diversion rings.
The products appear to have been purchased from unlicensed sources. Medications purchased from unlicensed sources may be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe.
PSM asks major online pharmacy sales platforms for information Today, the Partnership for Safe Medicines wrote to the leading online platforms that enable pharmacy-to-pharmacy drug sales to address a potential danger to the safety of the drug supply. PSM is concerned that counterfeit and diverted medication might be sold on online pharmacy-to-pharmacy platforms by criminals…
Cases of a botulism-like illness linked to cosmetic injections have been reported in Colorado, Illinois, Kentucky, Tennessee and Washington.
The state of Florida provided these FOIA-responsive documents to PSM for the state’s final and ultimately approved application to establish a Canadian drug importation program.
People in nine states sought medical treatment and some were hospitalized after receiving injections of fake Botox in what the CDC has characterized as “non-medical settings.”
Some employers are “saving money” on health insurance by hiring vendors to broker personal drug importation between their employees and unlicensed, illegal foreign pharmacies. Read more to understand the dangers of this cost-cutting practice.
Numbing creams that exceed the FDA-approved dose can cause irregular heartbeat, seizures and breathing difficulties. CBP has added FDA-regulated products to a pilot program to improve product traceability. Additional domestic and international news about medicine counterfeiting
State Senator Scott Wiener’s bill would eliminate under-reimbursement practices that create a dangerous opportunity for criminals to enter the legitimate supply chain.
A Las Vegas man was sentenced for money laundering as well as the illegal importation and sale of prescription opioids. Additional news across the U.S. and in India.
The Indian press reported that a Delhi-based ring sold fake cancer medicines in the U.S., China, and India. U.S. embassies and consulates in Mexico warned spring break travelers about counterfeit medicine. More domestic and international news about counterfeit medicines.
In spite of Etsy’s terms of use, there is a thriving trade in chemicals on the site, including those sold to individuals seeking homemade versions of highly regulated pharmaceuticals.
Colorado’s amended importation plan cuts prospective drugs by 78%. FDA Commissioner Califf warned about counterfeit medicines. A British couple who allegedly provided U.S. clinics they owned with illegally imported cancer medicines is facing extradition. Counterfeit medicine in Canada, Mexico and elsewhere.
Colorado’s updated Canadian drug importation application to the FDA cuts the drug list from 112 to 24 and adds Ozempic to the list of medicines it wants from Canadians.
The DEA put all e-commerce platforms on notice of their obligations whenever they facilitate transactions involving pill presses.
A Maryland drug distributor lost $2.7 million in a settlement over selling secondhand medicine to U.S. pharmacies. Additional news in Alabama, California, New Jersey, Tennessee and more.
Companies in New York and Florida were selling unapproved GLP-1 agonists. Operation Shield IV removed $69 million in illicit medicine and doping substances from European markets. A California man who distributed smuggled tianeptine received a two-year sentence.
PSM opposes the practice of reimbursing pharmacies below the cost of acquisition of medicine and supports efforts to reform reimbursement systems that eliminate this market dynamic that threatens the safety of our drug supply.
The government won prosecutions in Massachusetts and New Hampshire against defendants selling non-FDA approved drugs. A family warns about tianeptine. Pill presses in Texas, Virginia and West Virginia.
“We are deeply concerned about the impact importation of unapproved foreign prescription drugs will have on counterfeit medication in the United States, especially as FDA considers approving additional SIPs.”
DoJ alleged that eBay did not keep and submit required records to the DEA. Snap’s CEO spoke in support of the Cooper Davis Act. The FDA warned about copycat eye drops. Additional domestic and international news about fake and substandard medicine.
PBMs, by under reimbursing pharmacies, are creating a demand for pharmacies to seek lower priced medications even when they can’t exist at that price.. Criminals appear to be happy to become part of the supply chain.
The state of Maine opened it’s border to Canadian drug importation program for Maine residents. Dr. Kenneth McCall purchased and tested some of the medicines being advertised and sold to Maine residents and found them lacking in safety. Listen to his story.
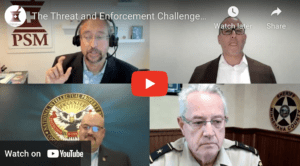
PSM’s Shabbir Imber Safdar sat down with three law enforcement experts about the problems they are seeing with counterfeit medicine. Watch here.
An explainer for the $59mm settlement between eBay and the Department of Justice over pill press sales. What does this mean for the problem of illegal pill presses? How is eBay responsible for what a buyer and a seller do on its platform?
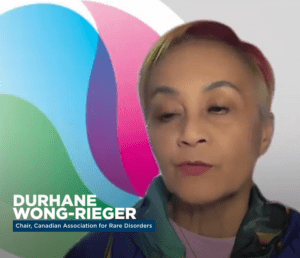
PSM’s Shabbir Imber Safdar speaks to Canadian patient advocate Durhane Wong-Rieger about the drug shortage situation in Canada.
What drugs have states said they plan to export from Canada’s drug supply? We combed all their applications to the FDA and compiled that list into one document.
Family advocates Andrea Thomas and Amy Neville will distribute naloxone at the Dirksen Senate Office Building. The VA will use the NABP’s digital DSCSA platform to help with compliance. Guilty pleas for dark web drug trafficking and manufacturing fake oxycodone, and more.
On January 17, 2024 PSM wrote the Senate Subcommittee on Intellectual Property to endorse S.2934, which would expand the Trademark Act of 1946 to extend liability for selling harmful counterfeits to online platforms. Read the letter here.
Tests of the counterfeits showed that they were made of many substances, including acetaminophen, duloxetine, caffeine and methamphetamine. Overseas, authorities seized counterfeit pregabalin in Belfast. Pakistan recalled toxic cough syrup. Fake Xanax trafficker Ryan Farace got an additional sentence for money laundering. News about pill presses in five states.
PSM recently published contracts, some obtained via FOIA, with key vendors implementing Colorado’s and Florida’s Canadian drug importation plan. Read them here.
Florida’s October 20, 2023 SIP application is the second to last application before FDA approval. The final application was filed on November 16, 2023.
Homeland Security seized over 1,800 pill presses in October and November 2023. The FDA warned Amazon not to sell supplements made with undeclared pharmaceuticals and issued another warning about toxic yellow oleander. More domestic and international news about counterfeit medicine.
PSM’s statement on the FDA decision and full coverage including media stories, official documents, and statements from stakeholders.
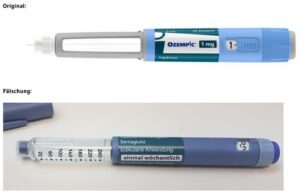
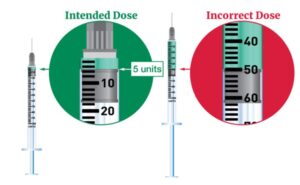
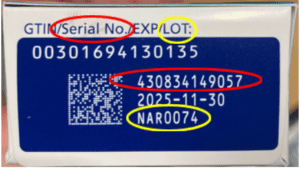
FDA continues to investigate counterfeit Ozempic (semaglutide) injection 1 milligram (mg) in the legitimate U.S. drug supply chain and has seized thousands of units of the product. FDA is aware of five adverse events from this lot.
This is a reprint of an FDA Alert. 8th Avenue Pharmacy Issues Voluntary Nationwide Recall of Notoginseng Formula Special Gout Granule Due to the Presence of Hidden Drug Ingredients, Diclofenac and Dexamethasone When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does…