Search
The Mexican Senate voted on March 12 to increase penalties to counterfeit drug sellers to nine years in prison and fines of up to 3 million pesos.
The Senate approved an amendment to article 464 of The General Health Law to increase penalties with 90 votes in favor. Senate Health Committee President Maki Esther Ortiz Dominguez said, “Anyone who sells or offers for sale, trades, distributes or transports medicine, drugs, raw materials that are falsified, altered, contaminated or adulterated, either in stores or in any other place….will be subject to the same penalty,” reported The News.
Read MoreAbstract Objective: To provide an overview of the counterfeit medication problem and recommendations of a joint American Pharmacists Association (APhA) Academy of Pharmaceutical Research and Science and APhA Academy of Pharmacy Practice and Management taskforce. Date sources: SciFinder and PubMed were searched from 1980 to March 2011 using the following keywords: counterfeit drug product, counterfeit medications, drug product authentication, drug…
Read MoreLast month, the United Nations Office on Drugs and Crime (UNODC) held a conference in Vienna to discuss the the production, distribution and trafficking of fake drugs by organized crime networks. The conference, which was held February 14-15, gathered an international group of experts from governments, law enforcement agencies, NGOs and the private sector to share information about the scale of the problem, which has significant impact worldwide. UNODC has found that in parts of Asia, Africa and Latin America as much as 30 percent of available drugs are fraudulent, and that the trafficking of unsafe or ineffective medicine is “a multi-billion dollar activity.”
Read MoreImage courtesy of INTERPOL.
Read MoreMarch
12, 2013 (Washington, D.C.) – The Partnership for Safe Medicines
(PSM), the leading advocacy organization dedicated to fighting the spread of
counterfeit drugs, applauds today’s
announcement of a bold new initiative between INTERPOL
and the pharmaceutical industry to combat the global health threat of
counterfeit and fake medicines.
This new initiative broadens the scope of the
successful Medical Product Counterfeiting and Pharmaceutical Crime Unit through
the creation of a Pharmaceutical Crime Program to assist and enhance worldwide
law enforcement efforts. Thomas Kubic, PSM Board Member and CEO of the
Pharmaceutical Security Institute, released the following statement hailing the
new agreement:
Question: What’s the difference between a Canadian retail pharmacy you can drive over the border to patronize and a “Canadian online pharmacy” that sends you medicine in the mail?
Answer: A Canadian web pharmacy is probably just a shipping company that claims to be a pharmacy, that may not even really be in Canada.
Question: Where do the medicines you get from a “Canadian online pharmacy” come from?
Answer: All around the world, but not from Canada.
The USFDA’s seized medications show that nearly half the imported drugs intercepted from four foreign countries were labeled as “Canadian.” But not only that, 85% of the medicines came from 27 countries around the globe, and tested drugs were found to be counterfeit. Learn more>
Question: Don’t Canadian online pharmacies provide great deals because they source medicine from safe Tier One countries like Canada, Australia and England?
Answer: No. Counterfeit medicine with no active ingredients, or cheaper and inappropriate ingredients, are the way they make the big bucks while tricking you into thinking youv’e gotten a great deal.
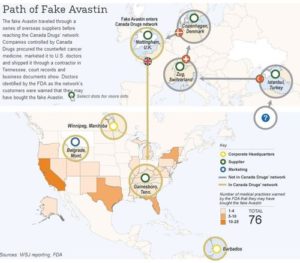
So-called Canadian web pharmacies (that actually hold no pharmacy license) assure customers that their drug imports are only from countries designated as Tier One by the Federal Food, Drug, and Cosmetic Act. However, the map to the left shows a selection of counterfeit medicine incidents that have occurred in Canada, Israel, and the European Union. Incidents have also occurred in the other Tier One countries not seen on the map. Some incidents have occurred in more than one country, such as the counterfeit cancer medication described above that traveled through several EU countries before it arrived in the US at the behest of a so-called Canadian online pharmacy. Learn more>
Question: Can’t the FDA and Health Canada protect me from danger if I buy medicines from outside the U.S.?
Answer: No.Says the FDA, “The FDA cannot help you if you have problems with medicine you get from outside U.S. regulation and oversight.”
Additionally medicines that are shipped between countries aren’t required to be inspected for authenticity. Health Canada isn’t going to check to make sure the medicines you receive are authentic, neither are the governments of any other country and that is most likely where your packages are coming from anyway.Learn more
Question: Isn’t the medicine I get from a “Canadian online pharmacy” just from the pile of medications that are price-fixed by the Canadian government? Aren’t they authentic Canadian medicines.
Answer: No. Those medications may never have even seen a maple leaf flag.
Even the even the Canadian government is warning their people about these websites. If they don’t want their people to buy medicine from so-called “Canadian online pharmacies,” how can you even begin to believe those drugs are legitimate?
Read MoreHow Did That Canadian Web Pharmacy Medicine Get to Me?
So called “Canadian” online pharmacies pretend to sell non-Canadians price-controlled medications for citizens. Evidence collected by the U.S. Food and Drug Administration questions their claim.
Read MoreView Master Map in a larger Are Tier One Countries Safe to Import Medicine From? No. Just because medicine purports to come from a supposedly safe country doesn’t mean that the medicine itself was either manufactured or inspected for safety by the country’s regulatory officials. Medicines can be transshipped through countries without ever having been opened or evaluated, with an…
Read MoreThough China has long been linked to the manufacture of counterfeit drugs, the last two years have shown that Chinese authorities are taking counterfeit drug crime much more seriously. China’s Food and Drug Administration (SFDA) cracked over 14,000 cases last year, a major toxic gel capsule ring was broken up, and Chinese authorities are working in concert with both the FDA and major drug manufacturers to track down counterfeit drug manufacturers and prosecute them. The founding of Partnership for Safe Medicines China also demonstrates China’s stakeholders’ commitment to improving patient safety both at home and abroad.
China made great strides in the last year in their efforts to combat pharmaceutical counterfeiting. Their cooperation with the US Food and Drug Administration on inspections is helping to safeguard both US and Chinese consumers. The founding of Partnership for Safe Medicines China underscores a new commitment to ensuing drug safety within their borders. Partnership for Safe Medicines China is the latest branch of the leading advocacy organization dedicated to fighting the global threat posed by counterfeit and misbranded drugs.
Read More