Search
An FDA alert warned that Vitafer-L has been found to contain undeclared tadalafil, an active pharmaceutical ingredient. Products containing tadalafil cannot be marketed as dietary supplements.
Read MoreThe U.S. Attorney’s Office in the Western District of Washington has charged two Indian nationals with selling Americans counterfeit Keytruda.
Read MoreThere has been controversy about the ad Hims & Hers ran during the Super Bowl. And now an SEC filing says investors should be warned about risk if their ads are found to be non-compliant.
Read MoreThis is a reprint of an FDA Alert.
Read MorePrescription Drug Affordability Board Activity through February 25, 2025 Activities Summary Colorado: Colorado’s PDAB did not meet in February. The next PDAB meeting will be March 7, 2025. Maryland: Maryland’s PDAB did not meet in February. The Board may have ad hoc meetings before its next scheduled meeting on March 24, 2025. Oregon: Board members met on February 19, 2025,…
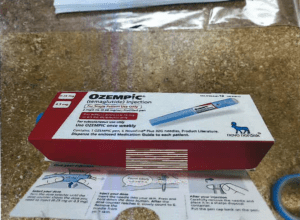
Read MorePSM’s new report shows that unregulated semaglutide and tirzepatide are slipping by FDA and CBP at the border. Semaglutide is no longer in shortage.
Read MoreThe Partnership for Safe Medicines today released a new report that found suspicious, unauthorized, and illegal ingredients for popular diabetes and obesity injectables (commonly known as weight loss drugs) are flooding into the U.S. from foreign sources despite U.S. laws forbidding them from coming through the border.
Read MoreThe Partnership for Safe Medicines applauds the Arkansas State Board of Pharmacy for utilizing new product verification technology for identifying illegitimate Ozempic quarantined by a pharmacy this week.
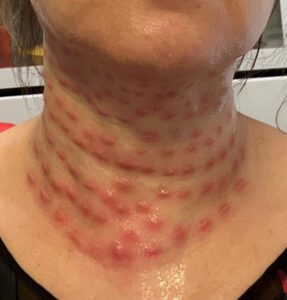
Read MoreMystery cosmetic injections allegedly harmed a client in Queens. PSM addressed a misleading drug ad for compounded weight loss medicine.
Read MoreYou could tell an American that they’re getting a compounded medicine and they would have no idea what that meant. That may be a legal disclosure, but that’s not good enough to protect patients who don’t know what the words mean.
Read More