Search
WHOIS data is searchable registrar information available for all websites on the Internet. It has long been used to trace criminal websites that host counterfeit and illicit drug sales, human trafficking, child pornography, and illicit and copyrighted content, as well as the websites of spammers, denial-of-services and phishing attackers, and other fraudsters.
Read More20-year-old Joshua Holton died of fentanyl poisoning in Tennessee after he took Xanax he’d bought online. He’d seen a TED talk online that suggested that users reviews were a reliable way to judge the quality of the drugs they were selling—but they weren’t.
Read MoreIn this editorial in The Globe and Mail, Ujjal Dosanjh, formerly a federal minister of health and a premier of British Columbia, explains that drug manufacturers have no incentive to sell Canadian provinces more medicine to fill the needs of U.S. residents. Importation will lead to drug shortages in Canada and counterfeit drug trafficking to the U.S.
Read MoreThis is a reprint of an FDA Alert. When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or the company. Company Announcement Date: February 24, 2020 FDA Publish Date: February 24, 2020 Product Type: Dietary Supplements Reason for Announcement: Product is…
Read MoreThe U.S. Food and Drug Administration (FDA) has just announced a successful joint operation with the Government of India targeting counterfeit prescription drugs, counterfeit over-the-counter medications, fake medical devices, and misbranded dietary supplements containing harmful ingredients.
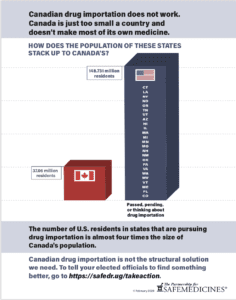
Read MoreThe number of U.S. residents in states that are considering importing drugs from Canada is almost four times Canada’s own population. Canadian drug importation is not the structural solution we need.
Read MoreDownload our “5 Ways” Consumer Bookmark for a quick reference about how to protect yourself from counterfeit medicine.
Read MoreThe following policy resolution was passed unanimously at the Jan. 8, 2020 board meeting in Washington DC. The governing board of the Partnership for Safe Medicines votes today to reiterate that the Partnership is organized to focus entirely of the safety of medicine as it travels through the supply chain, as we have since our founding sixteen years ago. To…
Read MoreResources for organizers on the HHS Canadian drug importation comment period Ways you can help spread the word Submit your own comments or tell people to go to http://safedr.ug/takeaction to submit their own. Email your community and ask them to comment. Follow us on Facebook and share our calls-to-action with your community. Resources Read PSM’s comment on HHS’s proposed rule…
Read MoreToday The Partnership for Safe Medicines filed comments with Health and Human Services about the dangers posed by its draft regulations for state-based Canadian drug importation programs. PSM cited historic problems with and patient harm from Canadian vendors selling counterfeit medications to U.S. patients and medical practices; showed broad opposition to the plan by Canadian stakeholders; and provides alternatives that don’t impact patient safety.
Read More