Search
If you are taking a life-saving HIV medication right now, or any kind of life saving medication, you may be concerned about how to be certain that your supply of medicine is secure. In this post, William Arnold of the Community Access National Network , PSM’s Shabbir Safdar, and Brandon M. Macsata of ADAP Advocacy Association offer suggestions to help.
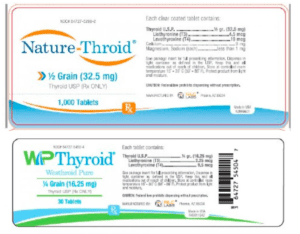
Read MoreRisk Statement: Patients being treated for hypothyroidism (underactive thyroid), who receive sub potent Nature-Throid® or WP Thyroid®, may experience signs and symptoms of hypothyroidism (underactive thyroid) which may include, fatigue, increased sensitivity to cold, constipation, dry skin, puffy face, hair loss, slow heart rate, depression, swelling of the thyroid gland and/or unexplained weight gain or difficulty losing weight.
Read MoreRisk Statement: Ingesting hand sanitizer, which is intended for topical use, may result in alcohol toxicity. Symptoms of alcohol toxicity may range from lack of coordination, slowed or slurred speech, drowsiness to coma, which can be fatal. Young children may experience a sharp decrease in blood sugar which may result in death. Pregnant women who ingest alcohol have experienced birth defects and developmental disabilities. Nursing mothers who ingest alcohol in above moderate levels may see developmental, growth and sleep pattern damages in their babies and may experience impaired judgement and ability to safely care for their child.
Read MoreIn this editorial, which was published in WBUR’s Cognoscenti on September 2, 2020, writer Sarah Ruth Bates explains why Canadian drug importation is too expensive and elaborate a solution to be effective.
Read MoreIn PSM’s round-up this week: ongoing coronavirus fraud, more hand sanitizer warnings and the week in fake medicine.
Read MoreDe‘Anna described her first experience with what she now knows was a counterfeit pill: “My first time taking a pill that was cut, a counterfeit pill, I blacked out, and woke up vomiting.” She also described losing her partner to a fake pill, who died from one when he was just 21.
Read MoreThis week’s “behind the scam” video discusses a real-life example of “Uplabeling,” which is a technique that counterfeit criminals have used in the past to make major profits. Learn about what happened when counterfeiters slipped diluted anemia medicine back into the legitimate drug supply.
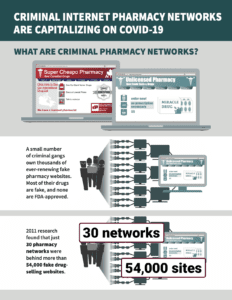
Read MoreIn PSM’s round-up this week: Our infographic summary of the NABP’s May report about fake pharmacies cashing in on COVID-19, ongoing coronavirus fraud, and the week in fake medicine.
Read More24-year-old Travis Jacobson was excited about an upcoming job interview. Recently graduated from Sacramento State University, he moved to Los Angeles to live with his best friend Landon and launch a career in public relations. Sadly, Travis never made it to that interview. Wanting a good night’s sleep beforehand, he took a Xanax pill that turned out to be a fake made with fentanyl, and it took his life.
Read MoreIn this editorial, Louisiana Attorney General Jeff Landry warns parents and students about the dangers posed by counterfeit pills being sold on college campuses.
Read More