Search
This editorial by Holly Strom and Kenneth Schell published in U.S. News and World Report on May 28, 2019 warns states considering drug importation that doing so will not keep costs down and also poses a safety risk to patients…
Read MoreFlorida’s Agency for Health Care Administration released a Request for Information for a Canadian Prescription Drug Importation Program. It specifically asked “The Agency is seeking information from entities with direct experience in coordinating all aspects of Canadian prescription drug importation. ” Responses are due June 25, 2019. Read the RFI
Read MoreDrug Importation in Florida: An Overview Current status: The U.S. Food and Drug Administration approved Florida’s Application to Import Prescription Drugs from Canada on January 5, 2023. Florida submitted its first application to HHS for permission to run a Canadian importation program in November 2020. After several amendments the FDA approved the program. (Read the FDA approval letter and the…
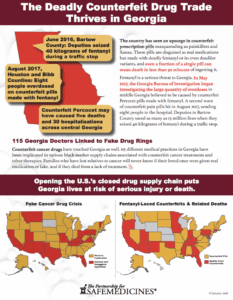
Read MoreA group of six people, five men and one woman all in their early twenties have been indicted on federal charges that they were using industrial pill presses to make counterfeit Xanax and fentanyl/carfentanil pills. They are also accused of selling the fake pills throughout Georgia via the Internet.
Read MoreWhat’s happening now? In an effort to try and reduce health care costs, the Governor and members of the Connecticut legislature have proposed a broad array of proposals to reduce the cost of health care for Connecticut residents. While PSM applauds efforts to reduce the cost of healthcare, proposals that circumvent the FDA and state board of pharmacy regulated supply…
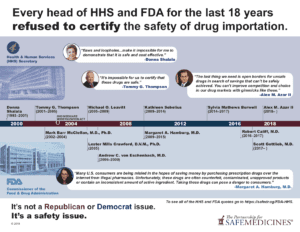
Read MoreRead their statements here. The Experts Agree: We Need to Protect Our Drug Supply Pharmacists, patient advocates, regulators, law enforcement and pharmaceutical companies and wholesalers share our concerns about the threat counterfeit drugs pose to American patients. Since 2000, not one head of the U.S. Department of Health and Human Services or one FDA Commissioner-no matter who appointed them-has been…
Read MoreIn this editorial, which was published in The Bend Bulletin on May 21, 2019, Canadian law enforcement veteran Don Bell explains why Oregonians can not rely on Canada for safe prescription drugs.
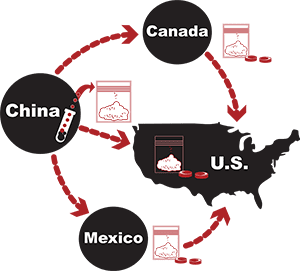
Read MoreDozens of the most highly qualified law enforcement officials and former, senior staff at the U.S. Food & Drug Administration have conducted in-depth analyses that show Canadian drug importation will lead to a massive increase in counterfeit drugs entering the U.S.
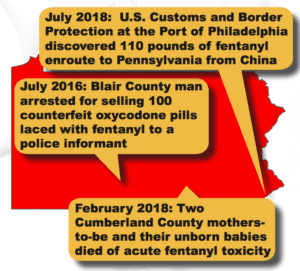
Read MorePSM started to track reported incidences of counterfeit pills made with fentanyl in October 2015. With the recent seizure in Kansas City, Kansas is the 47th state in which PSM has documented these deadly fake pills having been found in…
Read MoreKoledin ran a website called awakebrain dot com that marketed and sold misbranded and unapproved medication imported from Russia and China. It describes several instances when either the website or emails from Koledin described medications as FDA-approved, when in fact they were not.
Read More