Search:
Filter:
Sort:
On February 26, 2026, PSM was glad to see the Indiana legislature pass SB 282, a bill that strengthens compounding and medical spa regulation to keep Indiana patients safer.
As Europol seized a huge amounts of counterfeit pharmaceuticals abroad, a Kentucky legislator proposed a bill to regulate med spas after a constituent was seriously injured and adverse events related to weight loss drugs see surges across the US.
United States District Court Western District of Michigan Southern Division USA v Brandon Piper Felony Information February 19, 2026 Read the document.
PSM watches court cases that have an impact on drug supply chain safety. Click through to these cases to follow along with us.
Novartis and Genentech have filed a Lanham Act complaint against an alternative funding program and a Canadian pharmacy allegedly supplying Canadian Xolair to Michigan patients.
Washington State investigators found serious violations at a Mochi Health-affiliated compounding pharmacy producing GLP-1s, and a med spa bill in Indiana hears public testimony about a med spa bill.
Industry experts told us that these two companies are on the FDA’s Green List, despite problematic inspections in late 2024 and early 2025. Learn what the inspectors found.
Prescription Drug Affordability Board Activity, January 2026 Activities Summary Colorado: At its January meeting, the PDAB discussed ways to improve patient engagement and agreed to accept the dismissal of Amgen v. Mizner. The board’s next meeting will be February 20, 2026. Maryland: Maryland’s next PDAB meeting will be February 23, 2026 at 9:00 AM ET. Oregon: In January, Oregon’s PDAB…
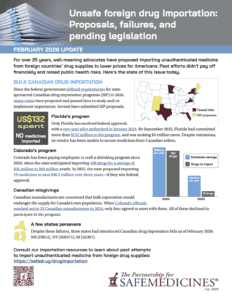
Well-meaning advocates continue to propose importing medicine from foreign countries to lower drug prices for Americans. Past efforts didn’t pay off financially and raised public health risks. Here’s the state of this issue today.
A Pennsylvania compounding pharmacy received another FDA warning letter following a series of enforcement actions, including a $1 million state fine in October.