Search
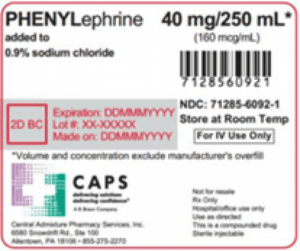
An FDA alert warned that Central Admixture Pharmacy Services issued a nationwide recall of Phenylephrine 40 mg added to 0.9% Sodium Chloride 250 mL in 250 mL Excel Bag due to the detection of black particulate matter in a single sealed vial of Phenylephrine Hydrochloride.
Read MoreAn FDA alert warned that Vitafer-L has been found to contain undeclared tadalafil, an active pharmaceutical ingredient. Products containing tadalafil cannot be marketed as dietary supplements.
Read MoreThe U.S. Attorney’s Office in the Western District of Washington has charged two Indian nationals with selling Americans counterfeit Keytruda.
Read More