Search:
Filter:
Sort:
Unregulated pill presses and molds make fake pills with dangerous and even deadly consequences for thousands of people every year. Read our July 2025 to January 2026 update about pill press seizures, policy developments, and legislation.
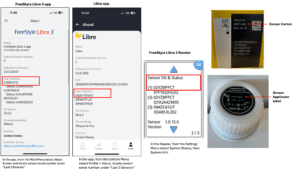
The FDA has posted a notice about the recall of continuous glucose monitors from Abbott.
The federal spending package that ended January’s government shutdown will prohibit PBMs from linking rebates to drug companies’ list prices. The FTC reported that PBMs inflated the prices of generic drugs.
United States District Court District of Massachusetts USA v Rodrigo De Medeiros Siqueira Affidavit Filed October 10, 2025 Read the document.
United States District Court District of Massachusetts USA v Rodrigo De Medeiros Siqueira Information Filed December 11, 2025 Read the document.
The FDA has posted a notice for a dietary supplement recalls involving products found to contain undeclared pharmaceutical ingredients.
PSM Executive Director Shabbir Imber Safdar released the following statement in response to news that Hims & Hers is now compounding pill versions of a newly approved GLP-1 weight-loss drug.
This post outlines practical steps Congress should take to strengthen pharmaceutical border security, reinforce regulatory oversight, and protect patients.
A patient at a North Dakota med spa told investigators she had to coach the clinic owner through the IV administration process. The owner allegedly presented herself as a registered nurse, despite holding no medical licenses.
Prescription Drug Affordability Board Activity, December 2025 Activities Summary Colorado: In October, Colorado’s PDAB heard testimony about its decision to set an upper payment limit on Enbrel, and set price of $600 per 50mg/1ml, effective January 1, 2027. The board’s next meeting is January 9, 2026. Maryland:The PDAB met on December 8, 2025 to review a draft of its annual…