Search:
Filter:
Sort:
The FDA has announced a voluntary nationwide recall of two dietary supplements sold exclusively online by www.umary-usa.com. Lab testing revealed that Unavy Ácido HIALURÓNICO and Umovy Ácido HIALURÓNICO contain undeclared active pharmaceutical ingredients: diclofenac, dexamethasone, and omeprazole.
Read MoreFederally-registered compounding facilities stopped making tirzepatide on May 22. This transition is significant for patients, and we at PSM think there are four things you should be watching for.
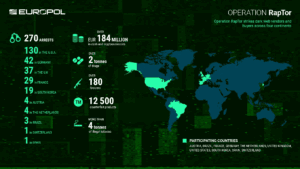
Read MoreOperation RapTor led to 270 arrests and the seizure of more than $200 million in currency and digital assets.
Read MoreArticles in Endpoints News and the Houston Chronicle raise questions about the regulatory process for 503B compounding facilities.
Read MoreExposes in the Houston Chronicle and Endpoints News examined Empower Pharmacy’s safety record. A grand jury indicted a Chinese company and three of its employees for pill press and counterfeit die mold sales.
Read MoreIn May 2025, the U.S. District Court in the Western District of Texas unsealed an indictment against a CapsulCN International and three Chinese nationals for their alleged role in the illegal importation of pill-making equipment.
Read MoreThe FDA wants to ensure that domestic and foreign companies receive the same level of regulatory oversight.
Catch up on research PSM released about medicines imported from unregistered sources.
Read MoreWhat if your blood thinners were made in an unverified location in Colombia? In March 2025, dozens of pharmaceutical shipments entered the U.S. that were manufactured in places no one would expect.
Read MorePharmaceutical border security Every day, inspectors from Customs and Border Protection, the Food and Drug Administration, and the Drug Enforcement Administration intercept scary medical products entering the U.S. This work is incredibly important to protect the safety of American patients and it’s difficult because criminals never stop innovating. PSM supports the work of border inspectors by commenting on proposed rules,…
Read More